Seminario INGEBI
Lunes 10 de Junio 13:20 hs.
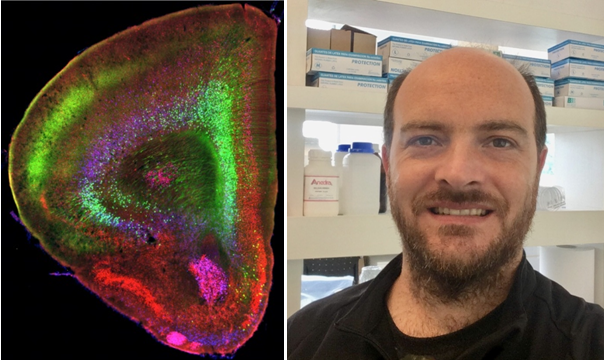
Dr. Mariano Soiza-Reilly
IFIBYNE, UBA-CONICET
“SSRIs target prefrontal to raphe circuits during development modulating synaptic connectivity and emotional behavior”
Antidepressants that block the serotonin transporter (SERT) such as the selective serotonin reuptake inhibitors (SSRIs) improve mood in adults but have paradoxical long-term effects when administered during perinatal periods, increasing the risk to develop anxiety and depression. The basis for this developmental effect is not known. We show that during an early postnatal period in mice SERT is transiently expressed in a subset of layer 5-6 pyramidal neurons of the prefrontal cortex (PFC). PFC-SERT+ neurons establish glutamatergic synapses with subcortical targets, including the serotonin and GABA neurons of the dorsal raphe nucleus (DRN). PFC-to-DRN circuits develop postnatally, coinciding with the period of PFC SERT expression. Complete or cortex-specific ablation of SERT increases the number of functional PFC glutamate synapses on both serotonin and GABA neurons in the DRN. This PFC-to-DRN hyperinnervation is replicated by early-life exposure to the SSRI fluoxetine that causes anxiety/depressive-like symptoms. We show that pharmacogenetic manipulation of PFC-SERT+ neuron activity bidirectionally modulates these symptoms, suggesting that PFC hypofunctionality has a causal role in the resulting altered responses to stress. Overall, our data identify specific PFC descending circuits that are targets of antidepressant drugs during development. We demonstrate that developmental expression of SERT in this subset of PFC neurons controls synaptic maturation of PFC-to-DRN circuits, and that remodeling of these circuits in early-life modulates behavioral responses to stress in adulthood.
